Visiting ‘Pharma Valley’ in Belgium
After the COVID pandemic we’ve become very aware of vaccines and their potential to keep us safe from infectious diseases. It’s also become a hot-button topic thanks to the rise of anti-vaxxers, who have their own beliefs about vaccines and their safety.
So when GlaxoSmithKline (GSK) invited The STAR to its research-and-development labs and vaccine factory in Belgium, I was eager to go and learn more about the biopharma company and its 140-year history of developing vaccines.
We first went to GSK’s Wavre site, which is about half an hour from Brussels. When we entered the secure gate, I was boggled by the complex’s size. Inaugurated in 1995, Wavre is the largest vaccine-manufacturing site in the world, equivalent to 70 football fields. GSK has another, smaller location in Rixensart, their historical site for vaccines that was inaugurated in the ’50s. GSK Belgium has around 9,000 employees, including 1,800 scientists from 80 countries.

“Two years ago, when we were preparing to split our consumer healthcare business to focus on the biopharma elements of the business, we set out our goals and we aim to positively impact the health of 2.5 billion people over the next 10 years,” says Madeleine Breckon, GSK’s vice president of Product and Pipeline Vaccines Communications and Government Affairs. “We have over 20 vaccines in our portfolio and 20 in our pipeline as well.”
GSK produced and delivered 500 million vaccine doses in 2022. Though they did not produce a COVID vaccine during the pandemic (a collaboration with French pharma company Sanofi fell through), around four in 10 children receive a GSK vaccine each year worldwide. “In the Access to Medicine index, GSK has consistently been number one, and that’s something we’re very, very proud of,” Breckon says.
GSK also deals in general and specialty medicines, focusing on infectious diseases, HIV treatment (they’re also looking into prevention), immunology/respiratory (they make Ventolin inhaler) and oncology. “In terms of deaths prevented, 3.5 to 5 million deaths are prevented globally every year by vaccination, and increasingly important is the estimated economic return, particularly for governments,” Breckon says. “We estimate around $52 returned for every dollar invested in vaccination, particularly in low- and middle-income countries.”
Two years ago, when we were preparing to split our consumer healthcare business to focus on the biopharma elements of the business, we set out our goals and we aim to positively impact the health of 2.5 billion people over the next 10 years,
Yan Sergerie, GSK’s vice president of Global Medical Portfolio Lead, who’s been with the company 15 years and is passionate about adult immunization, told a story about his dad, who suffered from a heart attack five or six years ago. “Now he’s more at risk of complication for infectious diseases, but it’s almost like he does not believe in them,” Sergerie says. He tried to convince him: “Do you know that if you get infected by influenza, it may increase your chances of having more inflammation in your veins and arteries, which could eventually lead to clogging? And if you clog your veins and arteries, what’s going to happen? Another heart attack. So if you get vaccinated against influenza, you may prevent that inflammation process and that heart attack. Truth be told, if you look in the literature now, vaccination against influenza will reduce by anywhere between 10% to sometimes 50% chances of developing heart attack.”

A week later Yan’s mom called him and said, “Your dad just took an appointment for his flu shot.”
While that was a victory for Sergerie, he notes that the global burden of infectious diseases remains high, in particular for herpes simplex virus (HSV), shingles, malaria, Clostridium difficile (a bacterium that causes diarrhea and inflammation of the colon), meningitis, chronic hepatitis B, Respiratory Syncytial Virus (RSV), and pneumococcal disease. “Vaccines induce immunity by imitating natural infections,” he says, “ and there is a lower tolerance of risk with vaccines than with medicines.”
He says that, beyond preventing disease, vaccines increase life expectancy and reduce work absenteeism and loss of productivity, “so you have more people paying more taxes, increasing your GDP. And when you’re older and healthy, you travel more, so you increase tourism as well. You relieve all of the pressure on the healthcare system. The role of the government is to understand this and start to consider vaccines not as a cost but rather, an investment.”

Healthy aging
A third of our life is spent as a senior citizen, according to Piyali Mukherjee, GSK vice president/head of Global Medical Affairs and Vaccines, and most of us want to spend this time productively. “In 2000, the average life expectancy around the world was about 66 years. Today, it’s about 73 years. By 2030 it’s going to be close to 80 years, and by the end of the century, a third of the population is going to be above the age of 60.”
She says vaccination can support healthy aging in older patients alongside other lifestyle factors like a healthy diet, exercise, and not smoking. Our immunity gradually weakens with age, so seniors—especially those with comorbidities—are at greater risk for hospitalization and death from flu, RSV (a common respiratory virus that causes mild, cold-like symptoms, but can be serious in infants and older adults), herpes zoster (for those who’ve had chickenpox, this virus hides and lies dormant in our nervous system unless reactivated, and this causes shingles) and post-herpetic neuralgia (pain that lingers after shingles).
“A number of vaccines are currently recommended for older adults to promote healthy aging, help avoid secondary complications and reduce the burden on healthcare systems,” Mukherjee says. “To help reduce the risk of mortality, pneumococcus, tetanus, diphtheria, hepatitis A and B, influenza and RSV. Some are primarily recommended to help preserve quality of life, like pertussis and shingles.”
The sleeping dragon awakes
Someone close to me had shingles, and it manifested on his right arm and shoulder as red blisters with a slight prickling sensation. It stopped spreading after one day, but he had to take antiviral medication for seven days to treat it.
“In Asia it’s called a dragon or a snake around the thorax—a blistering rash usually on one side of the body with small but exceedingly painful vesicles,” says Raunak Parikh, medical director of Global Medical Affairs for GSK. “Shingles, also known as Herpes Zoster (HZ), is caused by reactivation of the varicella zoster virus (VZV), the same virus that causes chickenpox (varicella).”
For those who had chickenpox at a young age, 90% of adults aged 50 years and above have VZV dormant in their nervous system, and one in three are at risk of developing shingles. “With age, as our immunity decreases, this virus has a chance to then reactivate,” Parikh says.
Shingles can have serious complications in immune-compromised patients like post-herpetic neuralgia and herpes zoster ophthalmicus, blisters in and around the eye area that may lead to vision loss in rare cases.
Belgian Pharma Valley
The next day we went to GSK’s research-and-development and vaccine-manufacturing site at the heart of Belgium’s “Pharma Valley” in Rixensart, Belgium, which is about half an hour’s drive from both Brussels and Wavre.
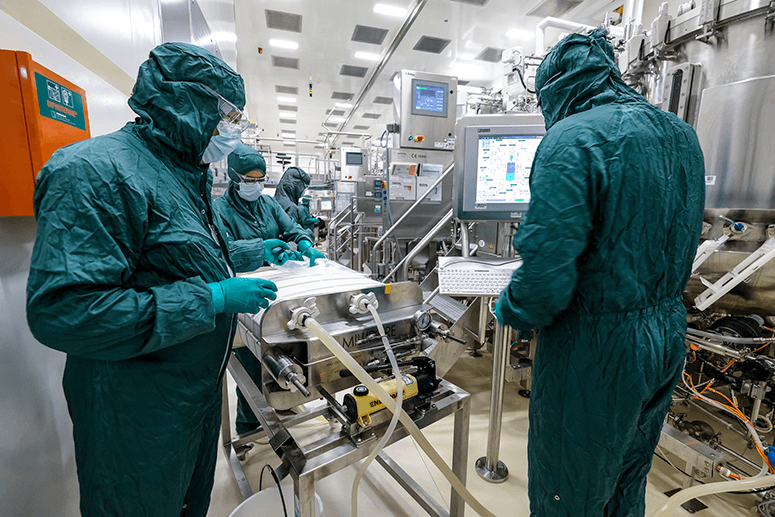
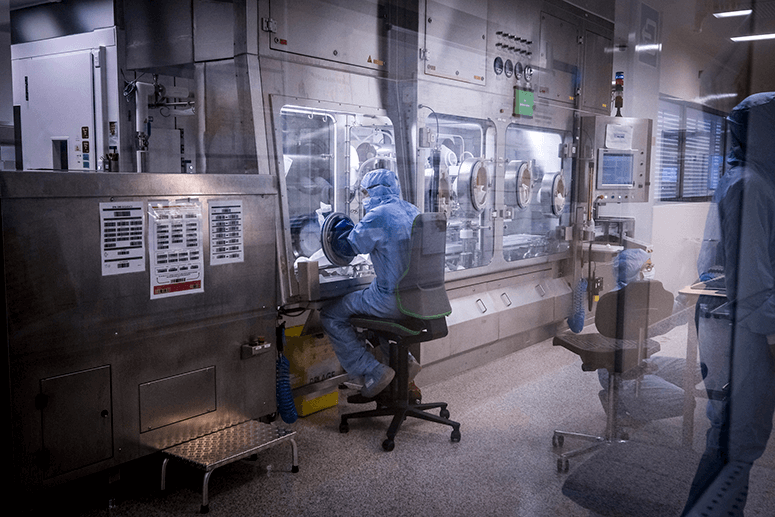
Rixensart has GSK’s largest R&D facility for vaccines, with two other centers located in Italy and the USA. In Rixensart’s vaccine factory, 200 people work five days a week during the day, because at night they clean the facility. Over 30 vaccines are made there, and they specialize in combining antigens (the toxins that induce an immune response in the body) with adjuvants (substances that boost the immunity of older or immunodeficient people and stabilize the product).
According to Yannick Vanloubbeeck, head of Immunology and R&D for GSK Belgium, there are two advantages to using adjuvants: “Firstly to boost immunogenicity, but also to decrease the quantity of antigen to protect the patient.”
At the R&D facility they ferment bacteria and grow them like vegetables in a garden to make soup (they use a lot of food metaphors here to explain processes), harvest the liquid and isolate the “white unicorn” (the antigen needed) through many filtration methods.
They do sterility testing every day to check that the facility is free from any contaminants, and at their Learning Center we experienced how hard it is to put on the protective gear necessary to work in up to six hours a day. Even washing hands is an elaborate process that puts our pandemic handwashing to shame.
In Wavre we also saw how they package the vaccines. Robots put the vaccines in boxes, tape them up and apply shipping labels (except for the Japanese market, which is paperless). It takes 30 minutes to load 5,000 doses onto one pallet.
Vaccine innovations
Historically in the 1800s, vaccines were made of live, attenuated pathogens, according to Vanloubbeeck. They would take the virus, weaken it and inject it whole directly into the patient. “This is how vaccinology started.”
This technique evolved until scientists could extract just the protein or polysaccharide they needed from the virus or bacterium, and this led to the development of subunit vaccines.
Continued evolution has led to gene-based vaccines, the most famous of which is the mRNA vaccine used against COVID. Now there are also DNA-based vaccines using viral vectors. “Viral vectors are viruses that are safe, they don’t produce the disease, but they produce a specific component of a bacteria or virus that you want in your vaccine,” Vanloubbeeck says. “The way I use to describe this is classical vaccines that are on the market today. When you inject them in the human body, you give your immune system a ‘meal,’ your immune cells will digest this meal and they will learn what they need to fight against. The next time they will see it through natural exposure with the virus or the bacteria. These new gene-based vaccines, you don’t give the meal to your immune system, you give them the recipe. So what the RNA does, the RNA enters your cells and basically tells your cells, ‘Produce this.’ And then your cells act as a manufacturing building and start producing the vaccine.”
For influenza, Vanloubbeeck says that since the flu virus changes every year, “in your vaccine, you include strains that are the most prevalent and circulating at that point in time.”
GSK has partnered with CureVac for joint development of multiple vaccine programs based on the latter’s second-generation mRNA platform. GSK also acquired Affinivax’s MAPS (Multiple Antigen Presenting System) technology to enable development of the next-generation vaccine candidates against invasive bacterial infections like pneumococcal disease.
These technologies will help address significant threats like gonorrhea (there are 80 million new cases globally each year, a 111% increase from 2009 to 2022 in the US), Clostridium difficile (a bacterium that causes 500,000 hospitalizations and 30,000 deaths in the US), and the Herpes Simplex Virus (HSV), which has infected 3.7 billion or 67% of people under the age of 50 globally with HSV-1, while 491 million or 13% of people aged 15-49 globally have HSV-2.
“Our pipeline is robust with 21 vaccine candidates in development to help address a range of potentially devastating diseases over the next five years,” Vanloubbeeck says. “By combining our expertise across a wide range of disciplines (e.g. immunology, virology, bacteriology, vaccinology) and innovation in life sciences, artificial intelligence, machine learning, and data analysis, we discover vaccines to make a transformational impact on people’s lives.”


