Here's a step-by-step guide on the government's vaccination plan
Health workers across the country are now preparing to embark on one of the most expansive vaccination programs in the country by next month that aims to achieve herd immunity in a year's time.
Health secretary Francisco Duque, however, told a hearing today, Jan. 18, by the House of Representatives Committee on Health, that the final scope of their roll-out will be mainly contingent on the availability of global supply.
“Makakamit natin ang herd immunity ngayong taon if there is enough global supply,” Health Sec. Francisco Duque III said.
Duque said they have already trained enough health workers, that will be deployed in teams, to start on the vaccination program’s implementation.
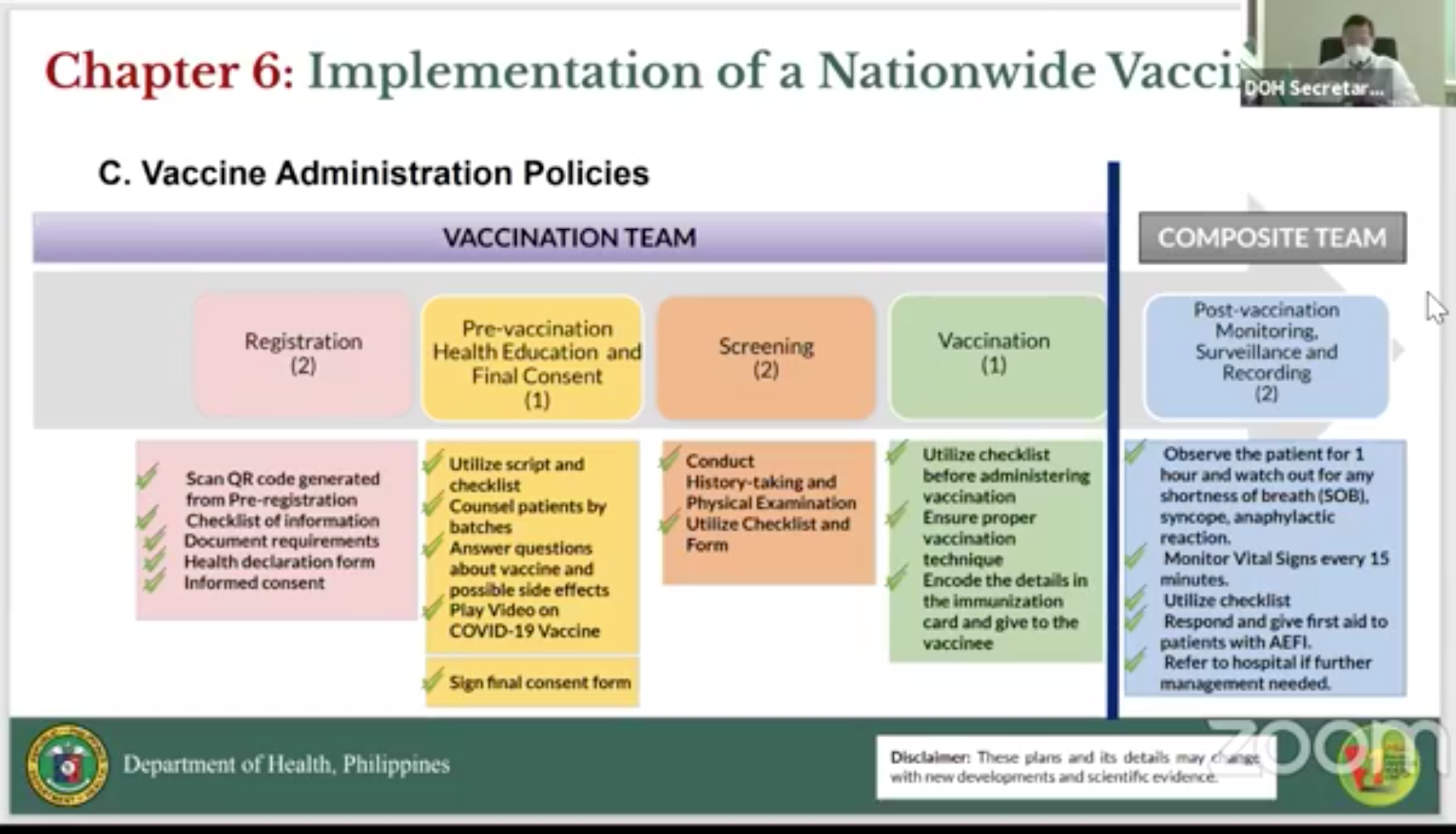
SOH presents details of implementation of the Nationwide Vaccination Program pic.twitter.com/4XhkIiksk5
— National Task Force Against COVID19 (@ntfcovid19ph) January 18, 2021
Each vaccination team of six will aim to inoculate a hundred people a day. The teams will be deployed in fixed vaccination sites in the country, which could be medical centers, hospitals, rural health clinics, or other government agencies.
The vaccination plan on the ground will be divided into five parts
1. Registration - QR code generation from pre-registration process, checklist of information, document requirements, health declaration form, informed consent;
2. Pre-vaccination - counselling patients, answering vaccine questions and possible side effects, instructional COVID-19 videos;
3. Screening - history-taking and physical examination,
4. Vaccination - checklist before vaccination, encoding details in vaccination card for the vaccinee
5. Post-vaccination monitoring - observing patient for one hour and monitoring of vitals every 15 minutes.
Duque said any adverse effect that will arise after the vaccination will be recorded and addressed through a mandated investigation protocol.
“We will identify the seriousness which will then be submitted to surveillance units,” said Duque.
Duque said that they are also talking with third-party providers for the distribution of vaccines that require storage of at least minus 70 degrees Celsius, such as the vaccine developed by Pfizer.
Furthermore, additional warehousing needs for vaccines will also need to be filled up by third-party firms starting August.
Duque, however, said that they are also retrofitting some government hubs to have storage capacity for additional storage capacity at low temperatures.
“Ngayon pa lang po ay ginagawa na po namin ito na several steps ahead for ensuring an effective and successful rollout plan,” Duque said.
"This is really quite a challenge, but pinaghahandaan po ng DOH," Duque said in reference to the entire vaccination strategy.


